There are a number of steps in the overall Canadian drug approval and reimbursement process.
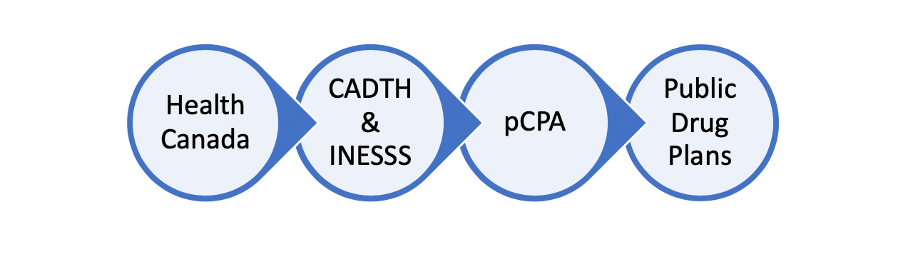
Step 1: Health Canada
Health Canada reviews drugs for safety, efficacy and quality before authorizing them for sale in Canada. Health Canada approved Luxturna in October
Step 2: CADTH & INESSS
In Canada there are two health technology assessment organizations which review the clinical and cost-effectiveness of a drug product; the Canadian Agency for Drugs and Technologies in Health (CADTH) and in Quebec, l’Institut national d’excellence en santé et en services sociaux (INESSS). CADTH and INESSS provide a recommendation to public drug plans on whether or not a drug should be reimbursed for public funding. CADTH and INESSS recommended that Luxturna should be publicly funded in November 2020.
Step 3: Price Negotiation
If a treatment has been recommended for funding by CADTH the provinces will then start to negotiate the price and the terms of sale with the pharmaceutical company. The pan-Canadian pharmaceutical Alliance (pCPA) is an alliance of provincial and federal governments who work together to negotiate these terms with the pharmaceutical company. The pCPA concluded negotiations with the pharmaceutical company in September 2022.
Step 4: Your Provincial Ministry of Health & Federal drug programs
Each province has their own Public Drug Plan. Once the p-CPA negotiation has been completed, each province makes a final decision to fund a drug.